The interplay between surface energy and wetting behavior is a fundamental aspect of materials science that has significant implications for various applications, from coatings to adhesives. Understanding how surface energy influences the way liquids interact with solids can inform the development of advanced materials tailored for specific functionalities. This article delves into the concepts of surface energy, wetting behavior, and adhesive properties while also highlighting the work of Droplet Lab, a pioneering organization founded by Dr. Alidad Amirfazli and his team at York University.
The Importance of Surface Energy
Surface energy, a critical property of materials, refers to the excess energy at the surface of a material compared to its bulk. It arises from the disruption of intermolecular bonds when a material is cut or formed, leading to an imbalance of forces at the surface. In essence, surface energy quantifies the tendency of a material to minimize its surface area. High surface energy materials, such as metals and glass, generally exhibit stronger intermolecular forces, while low surface energy materials, like Teflon or polyethylene, tend to have weaker forces. The amount of surface energy significantly impacts how a droplet of liquid behaves when it comes into contact with a solid surface, which is crucial in applications ranging from printing to painting.
Wetting Behavior Explained
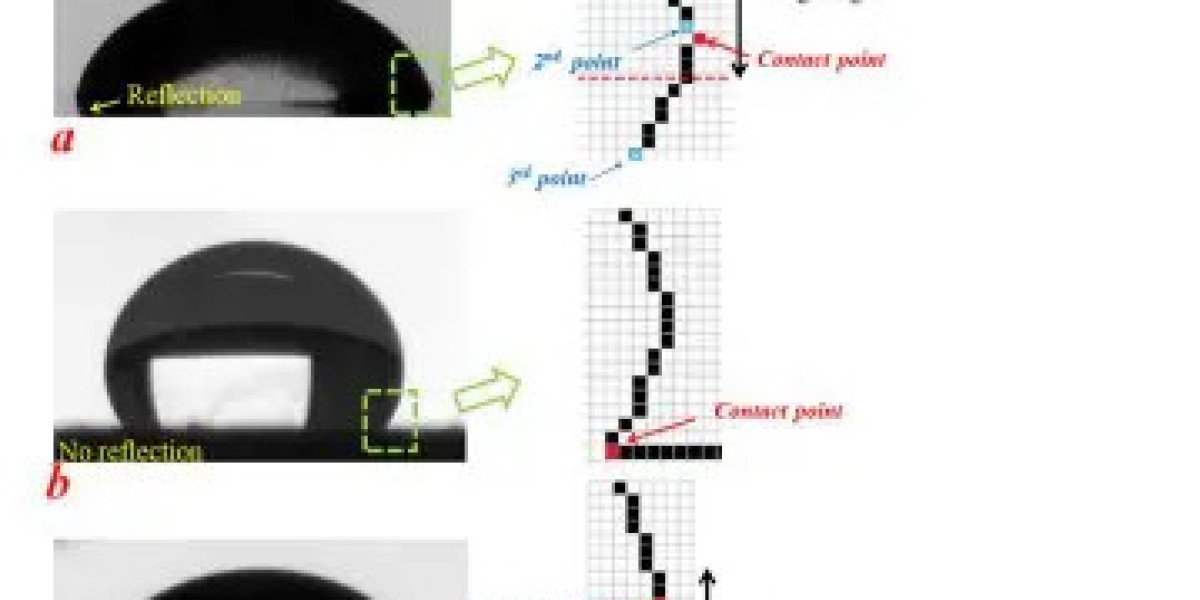
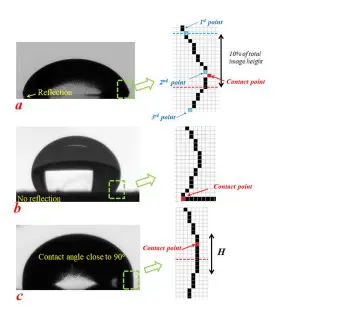
Wetting behavior refers to the ability of a liquid to maintain contact with a solid surface, which is contingent upon the balance between adhesive and cohesive forces. When a droplet of liquid is placed on a material, several interactions take place that determine whether the droplet will spread out or bead up. This behavior can be quantitatively described using the contact angle, which is formed at the three-phase boundary where the solid, liquid, and vapor meet. A contact angle of less than 90 degrees signifies good wetting, where the liquid spreads on the surface, while a contact angle above 90 degrees indicates poor wetting, where the liquid beads up.
The clean surface characteristics, such as roughness and cleanliness, alongside the inherent surface energy of the material, play crucial roles in determining the contact angle. For instance, a surface with high energy tends to attract liquids, reducing the contact angle and enhancing wetting properties. Conversely, low surface energy materials resist wetting, which can be undesirable in certain applications, such as adhesion processes where optimal surface bonding is essential.
The Role of Adhesive Properties
Adhesive properties hinge on surface chemistry and energy, influencing how well a material can bond with adhesives or other substances. When an adhesive is applied to a substrate, the effectiveness of the bonding process relies heavily on the material's surface energy. If the surface is poorly wetted by the adhesive, the bond strength will typically be compromised. For example, when applying glue to a low-energy surface, such as a plastic, the adhesive may form a weak bond, leading to failure under stress.
In contrast, high-energy surfaces, which allow for better wetting, facilitate stronger adhesive bonding. Manufacturers often tailor surface treatments or coatings to optimize surface energy for specific adhesive applications. Droplet Lab is at the forefront of this research, aiming to enhance our understanding of surface interactions and tailor materials with desirable adhesive properties.
Practical Applications of Surface Energy and Wetting Behavior
The insights gleaned from understanding surface energy and wetting behavior have practical implications across various industries. In the field of coatings, for instance, surfaces treated to enhance their energy can improve paint adhesion and durability. This is particularly crucial in automotive and aerospace industries where durability and resistance to environmental factors are critical.
In the realm of biomedicine, the interaction between biomaterials and biological fluids is vital for applications such as implantable devices and drug delivery systems. Manipulating surface energy can lead to enhanced biocompatibility, reducing the likelihood of adverse reactions in the body. Similarly, in the food industry, surface treatments can enhance the packaging materials' ability to resist moisture and contaminants, thereby prolonging shelf life.
Conclusion
The relationship between surface energy, wetting behavior, and adhesive properties is not only theoretically intriguing but also economically and practically significant. An increased understanding of these phenomena leads to the development of materials that can better meet the demands of various applications. Droplet Lab, through its innovative approaches and research, continues to shed light on these critical areas, underscoring the importance of surface science in advancing technology and material development. As industries innovate and evolve, the foundational principles of surface energy and wetting behavior will undoubtedly play a crucial role in shaping future applications in materials science.